How a model from the 1960s helped us revolutionize conventional cardiovascular diagnostics.
Measuring arteries directly
The Mission
The diagnostic shortcoming of traditional and inaccurate arterial diagnostics inspired and ultimately drove us to invent something better. We left well-trodden paths and developed a completely new and groundbreakingly different measurement method — with the potential to revolutionize conventional cardiovascular diagnostics.
Present “old school” cardiovascular diagnostics looks like that: Lacking precision in the measured values, thus also lacking reliability. Logically, the data obtained are then of very limited use for the early detection and prevention of cardiovascular diseases.
This serious deficiency of traditional cardiovascular diagnostics based on makeshift parameters provided the impetus for our mission — the development of a completely novel measurement method with previously unknown depth of precision.
The revolutionary idea behind it: An individualized simulation model providing insight into the actual arterial tree properties of any human being — without any intervention in the body, but nevertheless ‘directly’.

The original model by Abraham Noordergraaf and Nicolaas Westerhof
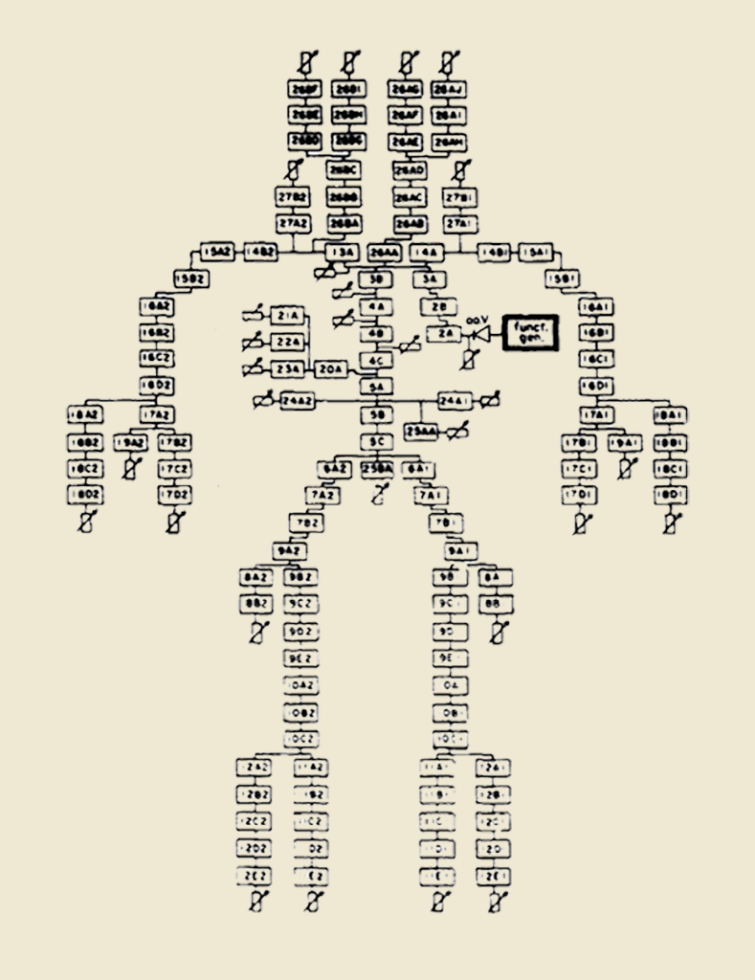
The starting point of all the following steps towards the development of a new measurement method within vascular diagnostics was working with a model. More precisely, we had to decide on a specific and already existing simulation model. The most important criterion: It had to be suitable as the basis for a modified arterial tree model that could be applied to any human. Amidst the plethora of models based on an analogy between the electrical circuit and the blood circuit, our choice fell on the model by Abraham Noordergraaf and Nicolaas Westerhof, published as early as 1969 at the University of Pennsylvania. The strength of this particular model lies, among other things, in the fact that it is well documented and quite relatable — thus, it formed the ideal basis for further developments.
Crucial corrections
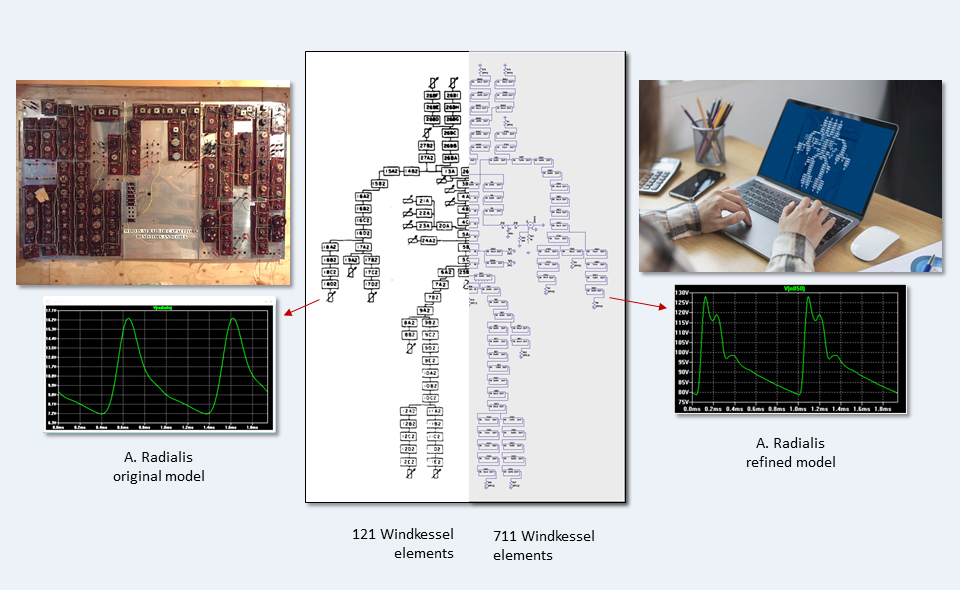
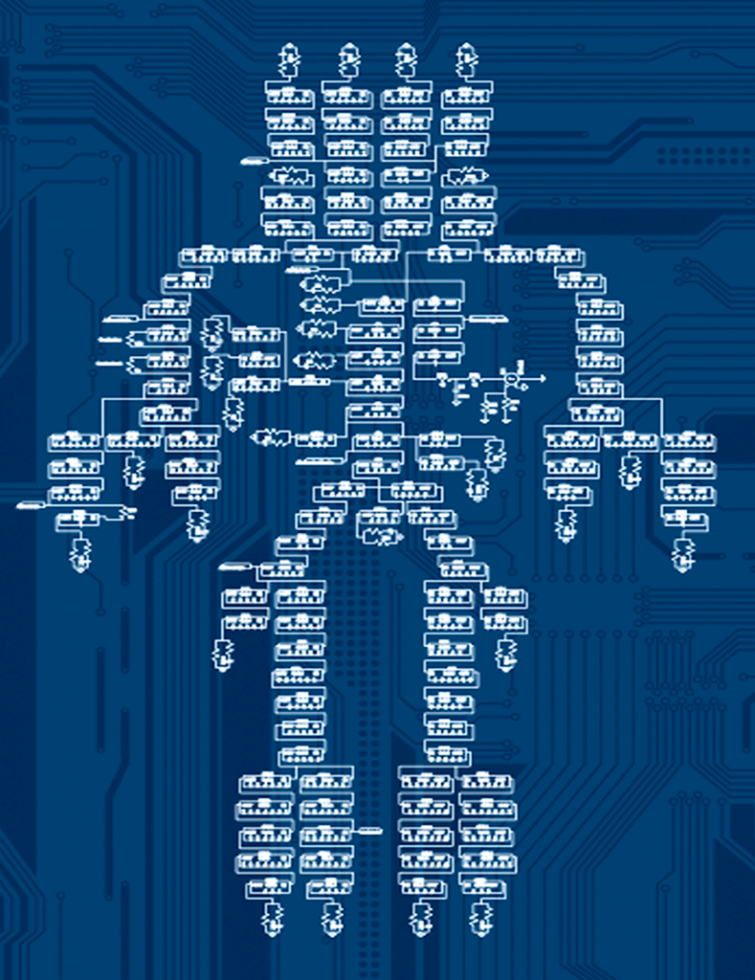
The original model had been laboriously wired by hand and had several simplifications in terms of reproducing the human arterial tree. In particular, individual arterial segments (windkessel elements) had been lumped together into larger units. In our simulations, we discovered that this led to a distorted reproduction of the actual flow properties in the arteries. We, therefore, resolved these simplifications in the virtual model replica with the aid of special software and thus achieved a significantly more refined, complex, and thus realistic representation of the arterial system.
The origin of the arterial avatar
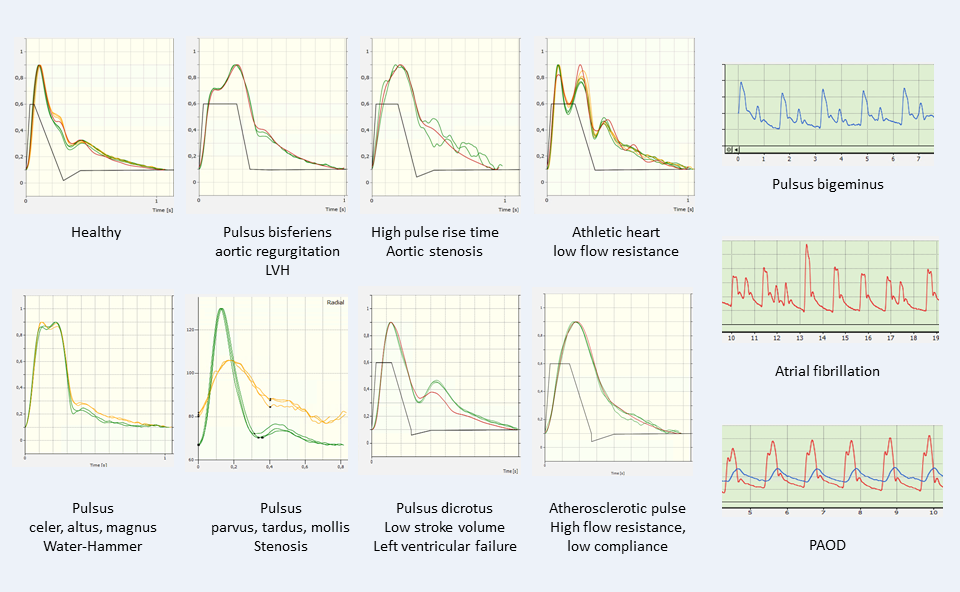
For illustrative purposes, we were able to reproduce typical pulse curve shapes in the virtual artery model, for example by simulating vessels set to ’narrow’ or ‘wide’. Next, we defined a total of eight system parameters. They are used to reproduce almost all pulse waveforms occurring in real people’s lives. These parameters are both based on a reasonable simulation time and a high simulation quality. Moreover, these parameters ultimately describe the actual arterial circulation properties, making them indispensable. Hence, they are also of high interest for clinical application.
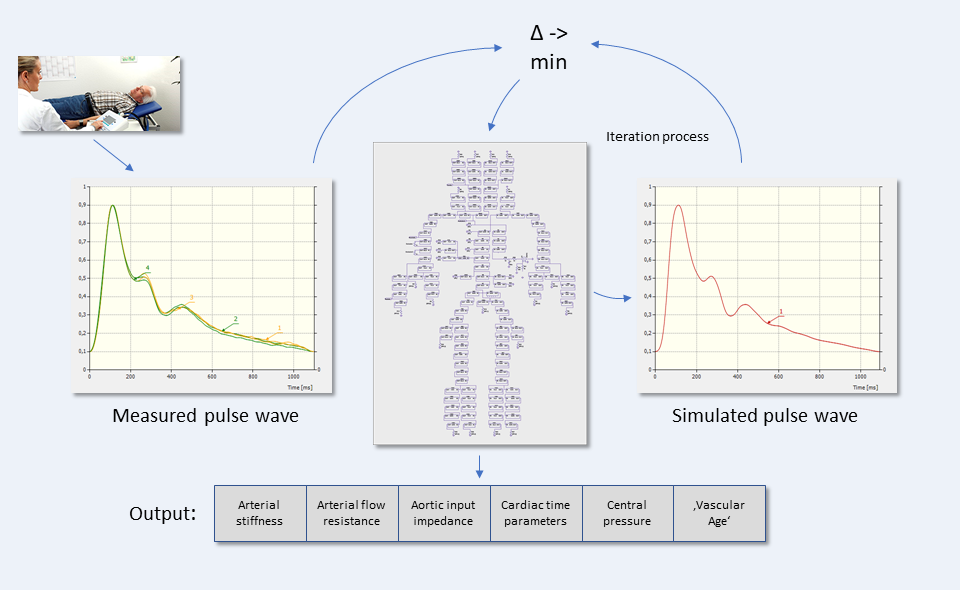
The final step was to develop a computational rule that adjusted our eight model parameters so that we ended up with the image of the arterial circulatory system of a real person. As a result, the data ‘output’ looks like this: The optimized model parameter set as well as the achieved simulation quality can be unmistakably read off in percentages via the software. In ‘badly’ simulated cases the quality reaches an accuracy of 97%, often 99.5%, but also 99.9%. At 100%, there would be a perfect match between simulation and reality.
The transformation of the original Westerhof model to the refined arterial avatar
The Model in operation
When the measurement of a patient with VASCASSIST is completed after ten to fifteen minutes, the ‘moment of truth has come after the finalization of three calculation stages of the underlying algorithm:
The patient’s vascular data, including the specific physical wall properties, can now be read at a glance based on the arterial tree model created during calculation: With such depth of detail and therefore at the same diagnostic level as if looking directly into the arteries. Using connected software, the recorded pulse waves can then be transferred to a desktop or notebook and visualized, ready to be analyzed in detail. Even the earliest vascular changes are detectable — in many cases, serious complications can thus be avoided through ideally rapid interventions. Many cardiovascular diseases are revealed during the software-supported visualization by striking deviations from the ’normal’, i.e. healthy, pulse curve shape.
From model individualization to different pulse waveforms
Sample calculation of a 65 year old’s arterial model