Model-Based Pulse Wave Analysis (mbPWA) — Taking a new path in cardiovascular diagnostics

Model-based Pulse Wave Analysis (mbPWA)
Taking a new path in cardiovascular diagnostics
Our disruptive cardiovascular diagnostic principle draws upon an arterial tree model from the 1960s. We refined it crucially and brought it to perfection. The convincing result: a diagnostic procedure that measures non-invasively yet directly — without any detours.
With the help of a virtually generated, realistic model of the human arterial vascular tree, we determine the central blood pressure via peripherally recorded pulse pressure curves. As precise as if you were looking directly into the arteries. Our diagnostic approach relies on an algorithm-based simulation of each patient’s actual arterial/cardiovascular condition. It works entirely without any falsifying surrogate parameters.
We challenge previous diagnostic approaches such as arterial applanation tonometry: Instead of an underlying population average, our data are based on actual, individual patient characteristics. For the first time, this enables us to obtain measurement results that visualize the current condition within the heart and the arteries on a 1:1 basis.
Our approach is nothing less than a breakthrough for diagnostics and therapy — and thus significantly improves patients’ quality of life: Doctors can detect and treat cardiovascular end-organ damage much earlier than previously possible. In addition, therapeutic interventions can be tracked with pinpoint accuracy and adjusted with much higher flexibility than ever before.
The Hemodynamic Background
Models are an essential tool whenever the goal is to simplify complex processes and illustrate underlying mechanisms and functions. When searching for exemplary images of the arterial system, one encounters a whole range of different explanatory approaches or constructs.
Human blood circulation models work with tube and distribution models based on the human anatomy. Simplified circuit models are another option. They trace blood flow and the propagation of the pressure wave generated by the heart through pulse waves (current pulse and pressure pulse). Against this background, the human arterial vascular tree can be recreated using so-called multicompartmental models and employing electrical components: In analogy to the blood flow in the circulatory system and the flow properties of electric current, one can work with the properties of resistor circuits R, inductor circuits L, and capacitor circuits C (RLC) to represent physical properties of the arteries.

A hardware-based primal model as the starting point of Model-based Pulse Wave Analysis (mbPWA)
A team of scientists led by Abraham Noordergraaf and, in further revision, Nicolaas Westerhof and collaborators first introduced a hardware-based, hand-wired approach to modeling the human arterial system in the 1960s in the United States. Earlier, this was primarily based on mathematical or physical models. In the final version of the model, a total of 113 hardware RLC circuits were interconnected to mimic the architecture of the arterial tree. Each segment represents about 2 to 8 cm of a vessel. While serially connected resistors and inductors mimic the conductance of an artery and the viscous and inertial properties of blood (longitudinal impedance), capacitors correspond to wall compliance (transverse impedance).
Technical Challenges
The operability and mechanical effort involved in the manual production of a passive electronic analog of the human arterial tree still posed a real challenge in the 1960s. Therefore, Noordergraaf’s model version and the version later revised by Westerhof included a whole series of technical concessions, in particular, the combination of individual windkessel elements (“lumping”) into one single component. Initially, the arterial properties (resistance, compliance, and blood mass inertia) were determined for 1‑cm arterial segments, which would have resulted in more than 700 windkessel elements for the model.
The original calculation base
Noordergraaf’s solution was to radically limit the immense manual effort that would have been involved in building 700 windkessel elements. He reduced their number in the final version to about 100 components the team had to assemble in hardware. Noordergraaf describes the relationship between the lumped R, L, and C values for each lumped size Δx versus the R’, L’, and C’ calculated for the 1 cm size using the following formula:
L = L’ Δx, R = R’ Δx, C = C’ Δx
The problem of reduced complexity
However, the model setup’s reduced complexity came at the cost of distorted reproduction of the natural flow properties: The coupling of windkessel units ignored the actual resonance properties of the arterial segments and their importance for transmitting the pulse wave. Noordergraaf was well aware of the height of the resonance peaks’ height distortion. However, he did not attach much importance to this fact because today’s modern simulation tools still needed to be developed. Westerhof later adopted Noordergraaf’s so-called lumped formulas in his improved model.
Our solution: The virtually refined Westerhof model
Using state-of-the-art software simulation tools, we could faithfully reproduce the model created by Noordergraaf, later revised by Westerhof and colleagues. During countless test runs, we simulated various typical arterial conditions. Thus, we gained essential insights into understanding the overall context and improvement of its behavior.
In the subsequent correction and refinement of the model — in contrast to Westerhof and Noordergraf — we attached particular importance to the preservation of the resonant frequencies, which we believed to be of dramatic importance: We preserved them in the new model within the individual 1cm arterial segments.
Thus, in the end, we completely removed the Noordergraaf-Westerhof model’s technological limitations. As a result, the overall model quality improved significantly: while the model refined by Westerhof consisted of 121 Windkessel elements, our refined model has 711 components. Consequently, our arterial tree simulation provides realistic pulse waveforms of the aorta and the brachial and radial arteries. The same is true for the true-to-life reproduction of various blood pressures.
The before-and-after effect: From the Noordergraaf/Westerhof model to the vascular avatar
Multiple coupled resonances instead of a reflected wave
The new arterial tree model yielded new insights regarding the origins of the physiological aortic-radial transfer function: We believe it is attributable to the coupling of many small resonance elements within a complex arterial tree.
This entirely new insight offers an alternative explanation and a significantly more plausible theoretical approach to the current (albeit controversial anyway) doctrine that attributes the secondary systolic peak to arterial pressure wave reflection. Our hypothesis of multiple coupled resonances within the arterial system is further supported by the fact that the refined model is not entirely new. The final version of our model merely corrected several simplifications in the original Westerhof model that had resulted in resonance frequencies that were much too low.
As our experiments with the refined model show, the aortic-radial transfer function evolves along the path from the ascending aorta to the radial artery. Similarly, the secondary systolic waves do not arise at specific distal sites but form along the entire way from the ascending aorta to the point where they are measured. Thus, for the first time, the formation of the known aortic-radial transfer function can be explained and demonstrated in an individualized arterial tree model.

The physiology of dynamic blood flow in carotid stenosis
A realistic image of the arteries
To understand the disruptive novelty of the refined model, we should consider the following: The original Noordergraaf/Westerhof model from the 1960s reproduced the arterial system of a 26-year-old man. Therefore, it was merely an object for visualization and study. However, the refined model, perfected through modern software tools, is the exact opposite of an idealized model of any human being. In contrast to a mere illustrative model, the new model is adaptable to the arterial tree of any real person.
This is a revolutionary, completely new approach, which consequently goes hand in hand with an equally innovative measurement method. As described above, the initial focus in the development of the new measurement method was on generating realistic pulse waveforms. Once this was achieved, the clinical benefits of the new model were immediately obvious: The artificial arterial system of the arterial tree model can now be adapted to a real patient. Thus, the newly created model provides valuable support in cardiovascular diagnostics and prevention in a variety of ways. The highlight: What could not be measured in real life without invasive measures (e.g. high-risk cardiac catheter examinations), can now be read without problems in simulatione, i.e. based on the image of a living person — in the vascular avatar.
Abnormal or pathological vascular conditions may manifest in increased conduction resistance R (e.g., stenosis, thrombosis, vascular tone dysfunction), increased inertial properties of blood L (e.g., high hematocrit), or low arterial compliance C (e.g., stiff or inflamed arteries due to arteriosclerosis or atherosclerosis). The individual multipliers dMult, lMult, and cMult obviously correspond very well with an individual’s specific arterial/cardiovascular health status.
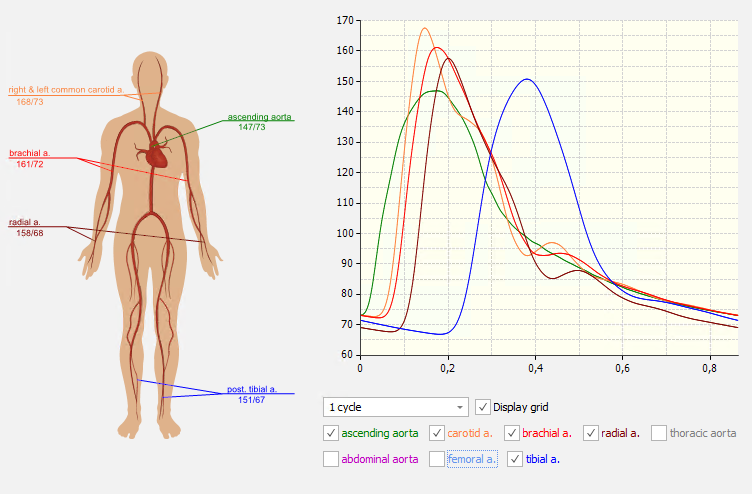
Evaluation of a Model-based Pulse Wave Analysis (mbPWA) measurement
Clinical Applications
Our insights concerning the resonant nature of the aortic-radial transfer function qualify for research and educational purposes. In addition, the individualized transfer function derived from our refined model incorporates different patient characteristics. Therefore, one can also apply it to determine central/aortic blood pressure (cBP) values.

Central vs. brachial: visualizing medication effects, adjusting therapies
Non-invasive measurements of central blood pressure are significantly instrumental in the clinical context. Our findings are of particular elevance when treating hypertensive patients: Upon determining their central blood pressure values, striking differences in the mode of action between different antihypertensive drugs become apparent. For instance, the antihypertensive effect of beta-blockers is often overestimated. This overestimation may be due to changes in arterial properties caused by bradycardia, increasing central systolic pressure, and pulse pressure. While further mechanistic studies are still pending in this regard, there is no doubt that our individualized arterial tree model is of great use for prospective, software-based investigations of various drug effects.
Aside from the fact that it is often necessary and in the patient’s best interest to reassess the prescription of beta-blockers, there is also an overarching, general benefit. Doctors can tailor any medical hypertension therapy to the individual needs and potential comorbidities of patients much more effectively than would be possible with purely brachial data by using the central, significantly more meaningful blood pressure values. Repeated control measurements during treatment allow physicians to ensure that desired therapeutic effects occur as expected — in the case of purely pharmacological intervention, for example, by changing drug classes, increasing or reducing doses, or using combination therapy. Measurements of aortic blood pressure also provide information on whether patients at an early stage of cardiovascular impairment can at least reduce an existing medication after consultation with the physician purely through effective lifestyle changes or even discontinue it entirely after some time. Provided patients stick to a health-promoting lifestyle, they can often avoid taking an antihypertensive medication altogether for a long time. Central blood pressure data (collected at regular intervals) are of crucial importance here, as they reflect the actual condition and hence, the heart’s functionality, in contrast to brachial-determined values.
Hemodynamic Parameters and Vascular Age

Invasive determination of central hemodynamic parameters showed an association between flexion time and augmentation index of the ascending aorta and the risk of coronary artery disease in symptomatic high-risk patients. These findings were confirmed noninvasively by applanation tonometry.
However, these conventional parameters represent merely indirect indicators of arterial properties. We postulate that the adaption factors used in the refined model directly correspond to the total arterial conduction resistance (dMult), blood inertia (lMult), and arterial wall compliance (cMult) of an individual patient. They represent different health states. Therefore, they are beneficial for estimating biological vascular age (often deviating significantly from the actual age).
An early warning system for endothelial damage

The ability to control vascular tone is impaired by aging and by endothelial dysfunction. This leads to hypertension and atherosclerosis. We hypothesize that such a dysbalance of vascular tone is also present at rest. This, in turn, is the basis of our hypothesis that increased conduction resistance detected with the refined model could be an early indicator of endothelial imbalance and atherosclerosis. This working hypothesis should be validated in a clinical trial, e.g. by measuring flow-mediated dilation (FMD) as the accepted gold standard.
In addition, we recommend further studies to evaluate the potential of the artificial artery model for risk prediction. Among other things, it could be used to guide the invasive assessment of patients at risk for premature coronary artery disease.